

Assume the change is reversible and the temperature remains constant. The Standard entropy change at equilibrium formula is defined as the thermodynamic quantity equivalent to the total difference between the entropy of a system is calculated using changeinentropy (Change in enthalpy +(2.303 R Temperature log10 (Equilibrium constant)))/ Temperature.
Standard entropy free#
Person: Was it trombone? No, Troubador.Determine the change in entropy (in J/K) of water when 425 kJ of heat is applied to it at 50 oC. Enthalpy, Entropy, and Free Energy Calculations Standard state values of G, symbolized as G, are commonly found in tables of thermodynamic quantities. Calculated Entropies and Integrated Heat Capacities at any temperature., Compare entropies for a given species. These are often (but not necessarily) chosen to be the standard temperature and pressure.In chemistry, the standard molar entropy is the entropy content of one mole of pure substancepure substanceHydrocarbon, a category of substances consisting only of hydrogen and carbon. Entropy data All data for a given species. Yes, cracking a stolen hash is faster, but it's not what the average user should worry about.) In chemistry, the standard molar entropy is the entropy content of one mole of pure substance at a standard state of pressure and any temperature of interest. (Plausible attack on a weak remote web service. The real standard entropy of ions in water is calculated from the temperature coefficient of the outer potential difference between a solution and the metal. (You can add a few more bits to account for the fact that this is only one of a few common formats.) Standard entropy change of formation, Sf is defined as the entropy of formation of 1 mole of compound from the elements present in the standard conditions.Sfcan be calculated for chemical compounds using the S values of elements from which the compound is formed. Standard entropy The absolute entropy of a mole of a substance at 1 atm and 25C The absolute entropy is difficult to determine, because it is difficult to determine a number of microstates corresponding to a particular macrostate. The term and the concept are used in diverse fields, from classical thermodynamics, where it was first recognized, to the microscopic description of nature in statistical physics, and to the principles of information theory. Uncommon (non-gibberish) base word ]Ĭaps? ]Ĭommon Substitutions ] Entropy is a scientific concept, as well as a measurable physical property that is most commonly associated with a state of disorder, randomness, or uncertainty. On each row, the first panel explains the breakdown of a password, the second panel shows how long it would take for a computer to guess, and the third panel provides an example scene showing someone trying to remember the password.)) Standard entropy of X2 Y2 and XY3 are 60 40 and 50 J K 1 mol 1 respectively For the reaction dfrac12X2 + dfrac32Y2 to XY3Delta H 30 kJ To be at equilibrium.
The comic is laid out with 6 panels arranged in a 3x2 grid. Entropy is maximum when the disorder is maximum. We have to find the order of increasing standard molar entropy.
Standard entropy password#
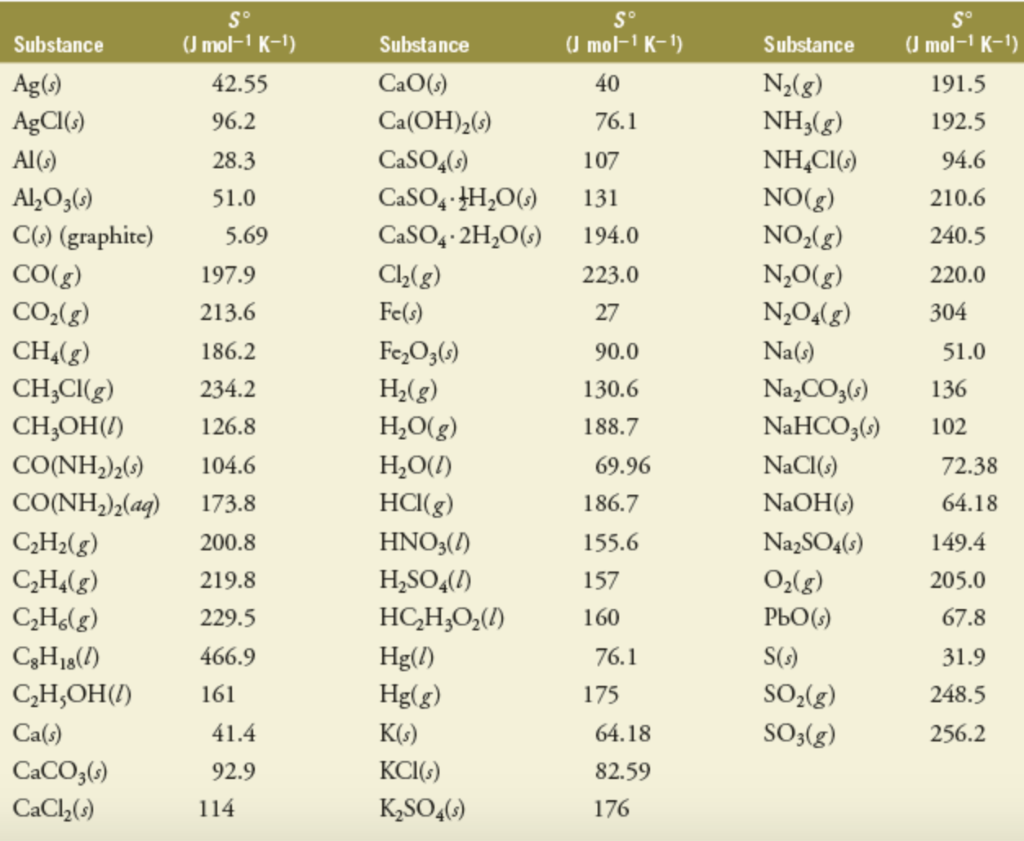
A set of boxes is used to indicate how many bits of entropy a section of the password provides. The given species are Na (s), NaCl (s), NaCl (aq). ((The comic illustrates the relative strength of passwords assuming basic knowledge of the system used to generate them. Standand Enthalpies of Formation & Standard Entropies of Common Compounds Substance State H f S (kJmol) (Jmol·K) Ag s 0 42.6 Ag+ aq 105.79 72.7 AgCl s 127.01 96.2 AgBr s 100.4 107.1 AgNO 3 s 124.4 140.


 0 kommentar(er)
0 kommentar(er)
